Current State of the Industry
Industry trends continue to demonstrate that slow
enrollment is the top issue plaguing clinical research.
80% of clinical trials experience delayed enrollment
48% never achieve the initial enrollment goal
How can pharmaceuticals, biotechs, and CROs mitigate the challenges related to slow enrollment and start-up delays in their clinical trials?
Why KUR Research?
With over 10 years of experience, KUR offers innovative solutions to address challenges in clinical research.
Our team delivers high-quality data to our sponsor partners, across all patient age groups, focusing on drug, device, and diagnostic development.
By building a network of various site types and specialties, we are able to meet virtually all sponsor needs through streamlined operations and a focus on quality.
-

Our Network
Our network is designed to be effective and efficient, and supports expedited patient enrollment. Our investigators attract and retain patients in clinical trials by building patient relationships organically, ensuring patients’ engagement, compliance, and retention throughout the trial. Our Investigative Site Network is augmented by KUR-trained and experienced site-level research staff who are focused on patient recruitment, protocol execution, compliance, and data quality.
-

Patient Management
Our proprietary database of patient information allows us to securely store patient data and efficiently search our database by age, gender, diangoses, and various inclusion/exclusion criteria. By filtering our database, we can provide sponsors with realistic and accurate enrollment data and tailor our clinical trials to the needs of our patients.
-

Quality Assurance and Data Management
Our dedicated 24/7 quality assurance team consistently monitors data to ensure data quality and integrity. KUR quality professionals remotely evaluate data collection and regulatory documents, review sponsor feedback from recent monitoring visits, and maintain equipment calibration documentation. We provide Sponsors and CROs access to an eSource system for QA and monitoring activities. The data is ready for source verification and external monitoring by the CRA within 24 hours of each subject visit.
-

Central Clinical Trials Management System
We are 95% digital and all clinical trial data is documented through our web-based Clinical Trial Management System (CTMS) to ensure quality, accuracy, and timeliness. With the rising need for remote capabilities within the research industry, our CTMS allows for seamless remote monitoring and real-time quality assurance auditing. By utilizing a CTMS, we can accurately track budgets, costs, and invoices to ensure an accurate financial outcome for each trial.
-

Central Marketing
KUR Research works closely with sponsors to ensure enrollment and retention goals are maintained throughout the duration of the study. One tool we utilize to combat enrollment hurdles is marketing and advertisement. By utilizing IRB-approved marketing materials, we reach qualifying subjects via a variety of digital, print, and social media sources. Marketing and recruitment efforts are done centrally to reduce the burden on the individual sites, so they can focus on protocol execution and data management.
-

One Company, One Solution
One of the many benefits of working with KUR Research is the streamlined process we utilize for study start-up. Sponsors can expect to execute the CTA and budget for all KUR sites through one department, and all regulatory start up documents will be handled and maintained by a KUR regulatory representative.
We understand that the start-up process is only the beginning of a long-term relationship with our sponsors, and KUR Research ensures an excellent experience from the start.
1. Study Start-up Process
Our Study Startup Process is based on the needs of the Sponsor. We are able to go from Site Selection to First Subject Enrolled in (as fast as) 10 days
- SIV Scheduling
- Source Document Creation
- Source Document Validation
- Budget & Contract Negotiations
- Regulatory Submission
2. Our Centralized Solutions:
Our Centralized Solutions are Designed to promote Quality and Consistency across Sites and Protocols by centralizing relevant processes to our corporate office
- CDA Execution
- Inform Consent Process
- AE/SAE Identification and Processing
- FQ Completion & Submission
- Staff Training
- Investigational Product & Supply Management
- Site Identification Feasibility
- Common SOP
- Temperature Monitoring
- Clinical Protocol Evaluation
- IT & CTMS Infrastructure
- Monitoring and Audit Support
- Protocol Management Assignment
- Clinical Site Biz. Dev. & Marketing
- Communication with CRO & Sponsors
- Budget Negotiations
- Quality Assurance / Quality Control
- Study Close Out
- Contract Negotiations
- Data Management / EDC Entry
- Patient Data and Site File Archiving
- Regulatory Activities
- Query Resolution
- Full AR/AP/Billing/Invoicing Cycle
- IRB/IEC Submission
- Subject Engagement & Recruitment
Acute & Chronic Conditions
Phases II to IV trials Adults and Pediatrics subjects
- Allergy/Immunology
- Cardiology
- Consumer Health
- Decentralized
- Dermatology
- Diagnostics
- Endocrinology/Metabolic Disorders
- Family Practice
- Vaccine
- Gastroenterology
- Infectious Disease
- Internal Medicine
- Medical Devices
- Nephrology
- Neurology
- Obesity
- OB-GYN
- Urology
- Orthopedics, Sports Medicine
- Pain Management
- Psychiatry
- Pulmonary Disease
- Respiratory
- Rheumatology
- Respiratory
- Sleep Medicine
- Smoking Cessation
Vaccines
Test new candidate vaccines
- Anthrax
- Avian Influenza
- Difficile (C-diff)
- Celiac Disease
- Chikungunya
- Cytomegalovirus (CMV)
- Dengue fever
- Diphtheria
- Ebola
- Encephalitis
- Zoster
- Parainfluenza
- Pertussis
- Plague
- Pneumococcal
- Rabies
- Respiratory syncytial virus (RSV)
- SARS-CoV-2 (COVID-19)
- Smallpox
- Tetanus
- Type 3 (PIV3)/RSV combo
- West Nile virus
- HCMV
- Hepatitis B
- Herpes
- Human papillomavirus (HPV)
- Human metapneumovirus (hMPV)
- Influenza
- Lyme disease
- Meningococcal
- mRNA vaccines
- Norovirus
- Zika virus
Sample & Diagnostic Trials
Collect High-quality biological specimens
- Via Specific sponsor protocol
- Via its sister company, Vexillum
Experts at the complexity, speed, quality and volume of subjects needed to successfully conduct Diagnostic Clinical Trials.
Specialty Population
- Geriatrics
- Women’s Health
- Pediatrics: Pediatric clinical trials are essential to develop age-specific therapies and intervention to provide the best medical treatment available. KUR Research has sites experienced with the specific ethical and clinical concerns of conducting trials within this special population, from newborn through adolescents.
Age Distribution
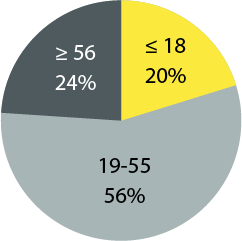
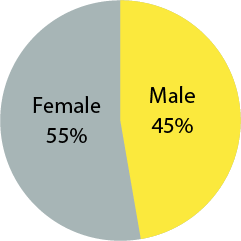
Sex Distribution
Ethnicity Distribution
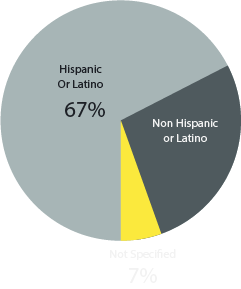
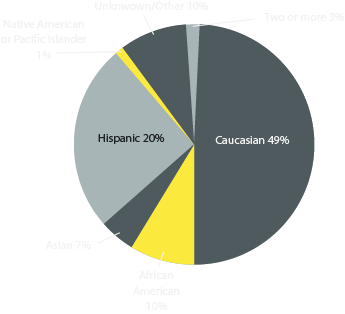
Race Distribution